Synthetic biology and genome editing are becoming more ubiquitous giving rise to the need of tracking alternations and distinguishing native from foreign DNA. CSIRO researchers are developing computational tools to detect genetic alterations.
One of the most humane ways of managing invasive species is to reduce “fitness” by altering an animal’s genome. Installing such a change in the entire population of the invasive species can be done very effectively using so called “Gene Drives”. However, before this technology can be applied to a wide range of real-world problems, scientists need to put in place effective safeguards that will restrict the desired genetic change to a target population. To help with this task, we are developing computational approaches for detecting whether the DNA sequence of an organism has been altered by Gene Drive systems. This provides an early warning system that can detect whether Gene Drives are about to escape from a target population.
Gene Drives – piggy-backing onto the “selfish” gene
Most genes are transmitted from one generation to the next with equal likelihood (i.e., 50:50). However, some genes are “selfish” and manage to bias their transmission with unequal likelihood (i.e., > 50%), thereby increasing their chance of being inherited. Through natural mating, selfish genes can spread through a population over many generations.
The advent of genome editing technologies such as CRISPR-Cas9, have made it possible to exploit the characteristics of selfish genes and engineer Gene Drive systems based on their biased inheritance. This enables Gene Drive to help with population management, where genetically modified animals would be released under appropriate regulatory oversight, and by breeding with wild animals the desired genetic change can be propagated through a population.
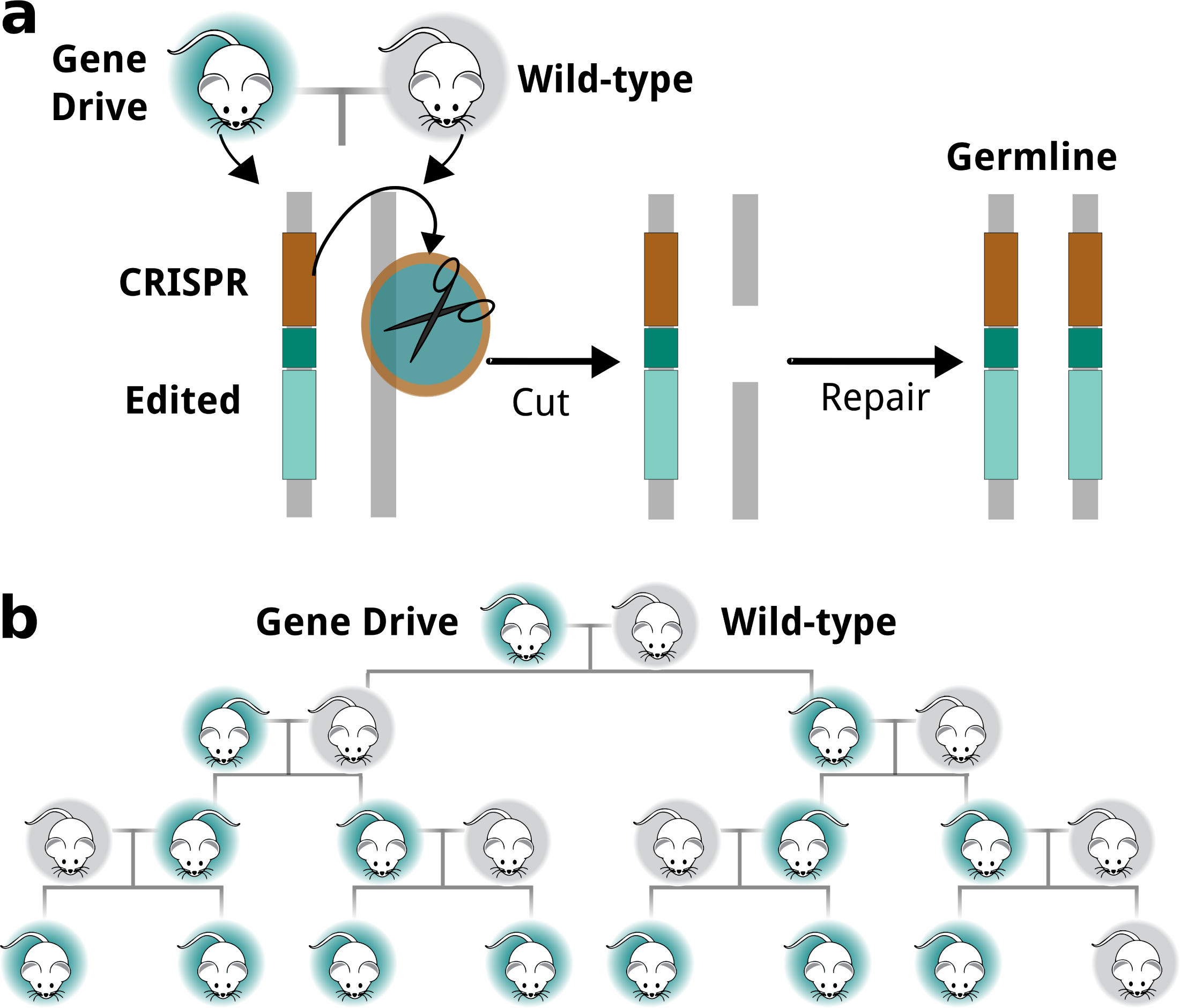
Gene Drives can help conservation efforts
Engineered Gene Drives hold great promise for managing wild populations (Kyrou et al. 2018 [3]). Gene Drives can be used to spread unfavourable genes for suppressing and eradicating invasive populations such as pest species (e.g., rodent pests) or disease carriers (e.g., malaria-carrying mosquitoes). One day, such technologies can also be used to spread beneficial genes (i.e., pathogen-resistant genes), thereby providing aid to endangered species and protecting them from becoming extinct.
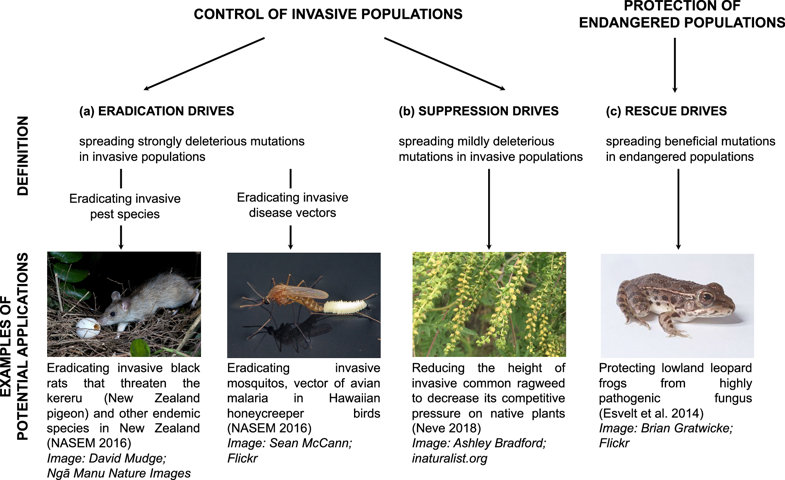
Mitigating risks pro-actively
Before this application can be applied to a wide range of real-world problems, methodologies need to be developed for detecting an unintended spread and reversing it if necessary. Specifically, releasing unchecked Gene Drive can have unintended consequences such as unwanted declines and species extinction. Another documented hazard is the potential escape and propagation of the desired genetic change through non-target populations.
Containing and controlling the spread of the desired genetic change to only the target population is crucial for managing some of the risks associated with Gene Drive and the release of genetically modified individuals into wild populations. However, determining whether individuals of a population have been genetically altered by an escaped Gene Drive is currently difficult.
Smart analytics to monitor Gene Drives
To improve traceability, we are developing an approach to detect whether the DNA sequence of an animal has been altered by an escaped Gene Drive. By analysing the DNA sequence of the individual and how its genomic content changes at different positions, we can distinguish between the native DNA sequence and that of a Gene Drive. The capacity to detect genetic alterations will therefore help us to prepare and effectively respond to the potential consequences of Gene Drives and help mitigate the risks associated with their use. Similarly, this technology helps with tracking the stability of the alteration through several generations, an important factor in assessing the ecological success of the intervention.


